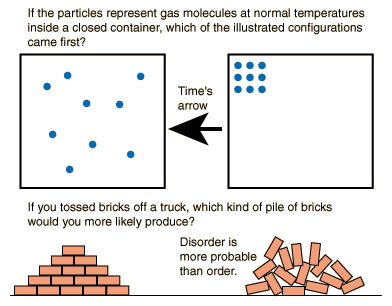
If there’s one concept that perennially defeats even students of physics or engineering it is entropy.
So let’s not go there.
Let’s talk about engines and heat pumps instead.
Don’t worry I’m not about to regale you with Coefficients of Performance or how big your radiators will need to be – though perhaps I will have to at some point. Let’s start here…
Heat pumps are like fridges run in reverse, right?
Wrong.
There’s no “reverse” in it.
A fridge is a heat pump; a heat pump is a fridge.
The devices are, in essence, the same. What differs is merely what bit of the device you consider to be the useful end. Both are covered by this diagram of energy flows,
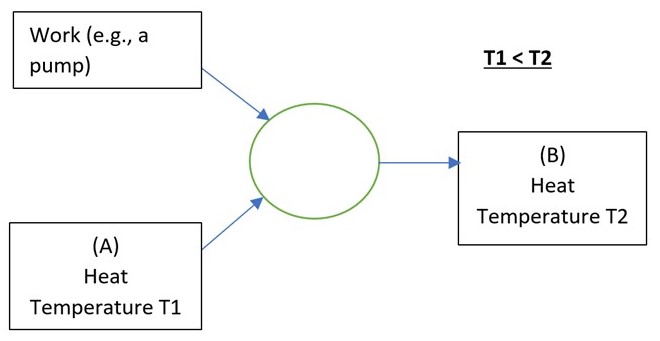
If it’s a fridge you want, then encase (A) in a cabinet called a “fridge”. It gets cold in there because you’re pumping the heat out. The heat gets dumped at (B), which is the atmosphere in your kitchen.
If it’s a space heating heat pump you want, then bury (A) in pipes in your garden and make (B) your radiators.
In both cases the mechanical pump, usually driven by an electric motor, is required because otherwise heat would not flow from cold to hot, would it? Left to its own devices, heat will flow from hot to cold, not the reverse.
There is an obvious analogy with pumping water uphill. If (B) is at a higher elevation than (A) it requires energy to drive a pump to move water from (A) to (B). But that is a treacherous analogy because pumping heat and pumping water differ in respect of the very phenomenon I am trying to illustrate.
Pumping water is, in principle, fully reversible. The energy you supplied in the form of electricity to do the initial pumping (and hence to increase the water’s gravitational potential energy) can, in principle, be regained in full by allowing the water to flow back to (A) through a turbogenerator. This is exactly what pumped storage power stations do. They are not 100% efficient in practice, of course, as motors and pumps are never loss-free and losses through fluid turbulence are unavoidable. But that’s not the point. The point is that there is no theoretical limit to the technological improvements that could, in principle, be made towards 100% efficiency.
In the case of heat, however, that is emphatically not the case.
What happens if we reverse the pumping of heat?
The reverse of a heat pump is a heat engine
For a heat engine the energy flow diagram is identical to that above except for reversing the direction of all the arrows,
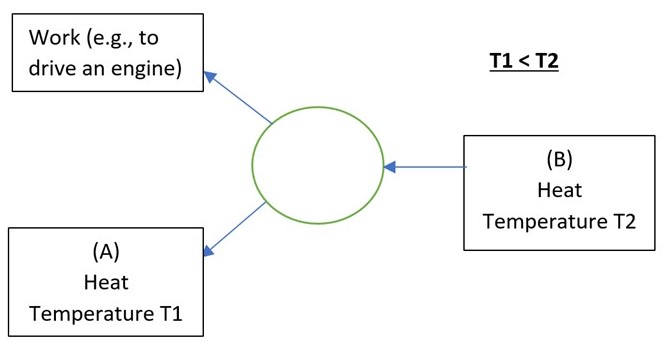
Heat is now flowing naturally in the direction it likes, from hot to cold. In the process we can tap some of this heat energy and convert it to useful work, e.g., to drive your car. The key word here is some. There is a theoretical upper limit to the proportion of heat extracted from temperature T2 and deposited to temperature T1 that can be converted to work. It is given by,
Maximum Efficiency of a Heat Engine = (T2 – T1) / T2 (absolute temperatures, K)
So 100% efficiency is achievable only if the cold sink is at absolute zero (impossible) or the hot sink is at infinite temperature (impossible).
Not all energy is the same
This is the nub of the matter. It is why this, rather embarrassingly pedagogic, post is a useful precursor to the next (The Iniquities of Gas Generation).
These days you will have noticed that your domestic energy bill expresses both your electricity usage and your gas usage in units kWhr (kilowatt-hours). The same unit can be used for both because both are measures of energy. You could equally use for both: Joules or ergs or calories or Btu or tonnes of oil equivalent (all of which you can find in publications).
But you will also notice that (despite recent eye-watering price hikes) gas is charged at less than one-third the price of electricity per kWhr. If all there was to these commodities was “energy”, as measured by kWhr or Joules, why would this be?
It is extremely obvious that gas and electricity are not interchangeable. There is more to these commodities than just their energy content. Leaving aside more exotic industrial processes, what can you do with gas at home other than burn it? Gas provides heat, full stop. Electricity, however, provides work.
You could (and perhaps do) power your oven and your hob with gas, and use a hob-based kettle, because these things need heat.
But you cannot (directly) power your TV, your PC, your mobile, your vacuum cleaner, your power tools, your automatic garage door or your electric car with gas. They require the provision of work or its equivalent in the form of electrical energy. You could install your own gas powered mini-power station at home, but you’d be lucky if it were 33% efficient, so you’d not save any money that way.
[Aside for pedants: Rather annoyingly for the cleanliness of my argument there are gas-powered fridges. It’s left as an exercise for the reader to work out why such devices do not undermine my message in this post. And as for lighting, gas mantles are fiddly things and nowhere near as effective as electrical lighting. There’s a good reason why electric lights displaced gas mantles so very quickly in the Victorian era. Also, gas mantles are staggeringly inefficient].
The uselessness of gas to power your electrical devices is a manifestation of a quality of electrical energy which is absent from gas (or, more strictly, heat). It is a quality independent of energy and not measured by kWhr or Joules. It is closely related to our banned word, “entropy”. It is also closely related to another thermodynamic concept: free energy. Unfortunately the use of that term outside of scientific papers must surely beg misunderstanding like no other.
The take-home message, however, is simple: not all energy is the same. The worth and usefulness of an energy-commodity is not measured by energy alone but also by a type of “quality” which is never quantified in the commercial context but is implicit in the specific commodity type. Electricity is a higher quality energy source than gas; that’s why it’s more expensive, and that’s why it’s more versatile in use.
The nature of this “quality” is that it cannot be created from nothing but it can be degraded and reduced. That’s why you can convert high quality energy (work, electrical energy or potential energy) to low quality energy (e.g., heat) with 100% efficiency. That involves reducing the “quality”, which is possible – indeed, ultimately unavoidable. But you cannot fully convert low quality energy (heat) to high quality energy (electricity, etc) because that would mean an increase in overall quality, which is forbidden (specifically by the second law of thermodynamics).
And that’s why it might just be iniquitous to generate electricity from gas (see next post). In doing so we are obliged to throw away energy, in the form of waste heat (point A in the above diagram for the heat engine) – whereas the supply of gas to consumers and their direct usage of its heat of combustion would waste nothing (in principle).
[Experts will shudder at my horribly informal, and frankly imprecise, description. They will note, however, that my “quality” is the negative of entropy].
The Three Laws of Thermodynamics
The whimsical version of the three laws is,
First Law (essentially the conservation of energy): You can’t win, you can only draw.
Second Law (entropy cannot decrease in a closed system and increases in irreversible processes): You can’t even draw, only lose.
Third Law (absolute zero is unobtainable): You can’t get out of the game.
And they say that Malthus was a misery!